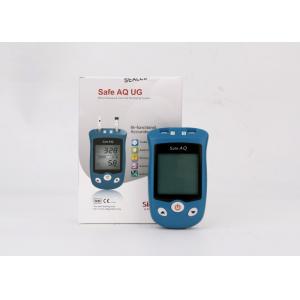
Product Details
Alternate Site Testing Electronic Glucose
Monitoring System , Diabetes Blood Sugar
Monitor
Specifications:
TEST FUNCTION | BLOOD GLUCOSE | URIC ACID |
|---|
Test method |
FAD-GDH |
oxidized ferrocene derivative |
Sample type |
peripheral capillary whole blood
/venous whole blood |
peripheral capillary whole blood
/venous whole blood |
Blood sample |
0.6ul |
3ul |
Test range |
20-600mg/dL (1.1-33.3mmol/L) |
3-20mg/dL |
Test time |
5s |
25s |
Memory |
100 sets |
100 sets |
Strip validity |
18 months |
12 months |
Tips
1.Do not use for neonates (newborns or infants).
2.Do not use to screen or diagnose diabetes mellitus.
3.Do not use for critically patients.
4.All parts of the Safe-Accu kit should be treated as biohazard and
can transmit infectious
diseases, even after you clean and disinfect.
5.Keep test strip vial away from children. A child could choke on the cap or be harmed if the
drying agent in vial is swallowed, inhaled, or contacts skin.
ABOUT SINOCARE INC.
Sinocare Inc. is a high-tech company. We have been fully dedicated
to the innovation of biosensor technology, developing,
manufacturing and marketing rapid diagnosis testing products for
people with chronic diseases and healthcare professionals.
We love, so we focus. “Pour Love into Work”, Sinocare provides high
quality products and services for people with diabetes and other
chronic diseases to help them improve their life quality. We
sincerely invite you to join us, explore your opportunities of
advancement in Sinocare.
World Leading BGMS company. Products sold to 135 countries.
We are now undergoing a great change of disease spectrum. In recent
years, diabetes, cardiovascular or cerebrovascular diseases, tumor
and other chronic diseasesbacame a great threat to our health and
economic systems. more than 75% of their healthcare in the world
expenses on the treatment of chronic diseases. Growing incidences
of chronic diseases not only burden our society with huge cost on
healthcare, but also seriously reduce the quality of life of
patients with chronic diseases.
We Helps to improve the quality of life in patients with chronic
diseases such as diabetes
Company Profile
Sinocare is a high-tech enterprise dedicated to the development,
production and sales of the rapid detection of chronic diseases in
the use of bio sensing technology. Since its inception in 2002, has
been adhering to the business purpose “Honor Commitment Dedicate to
Healthcare", focus on promoting the development of diabetes health.
Sinocare is a high-tech enterprise dedicated to the development,
production and sales of the rapid detection of chronic diseases in
the use of bio sensing technology. Since its inception in 2002, has
been adhering to the business purpose “Honor Commitment Dedicate to
Healthcare", focus on promoting the development of diabetes health.
Sinocare as a national high-tech industrialization demonstration
project, has won National Innovation Fund support, and took the
lead through the ISO 13485 Quality Management System certification
and the CE certification of EU. The SANNUO series blood glucose
meter and a test strips, with the characteristics of the "accuracy,
simple and economic", recognized by the vast number of consumers,
the "Sinocare" trademark was identified as "China well known
trademark by State Administration of industry and Commerce of. The
products are not only popular in China, but also in the
international market. In March 19, 2012, the company listed on the
Shenzhen stock exchange gem, as the first Bio sensor stock in
China. In 2015, the blood glucose meter in the domestic retail
market share reached 51%, becoming the industry leader in blood
glucose monitoring products, the same year was elected to the
Forbes Asian small and medium companies top 200 list. In 2014
September,Sinocare acquired Beijing Jianheng Diabetes Hospital,to
step into health service area. In January of 2016, Sinocare
acquired Nipro diagnostic Inc. (now renamed as Trividia Health
Inc.), the world's sixth BGM enterprise, and reached the
international leading BGM corporation, In July of 2016, completed
acquisition of PTS Diagnostics, expand the POCT testing business,
to provide a more comprehensive solution for chronic disease
prevention.